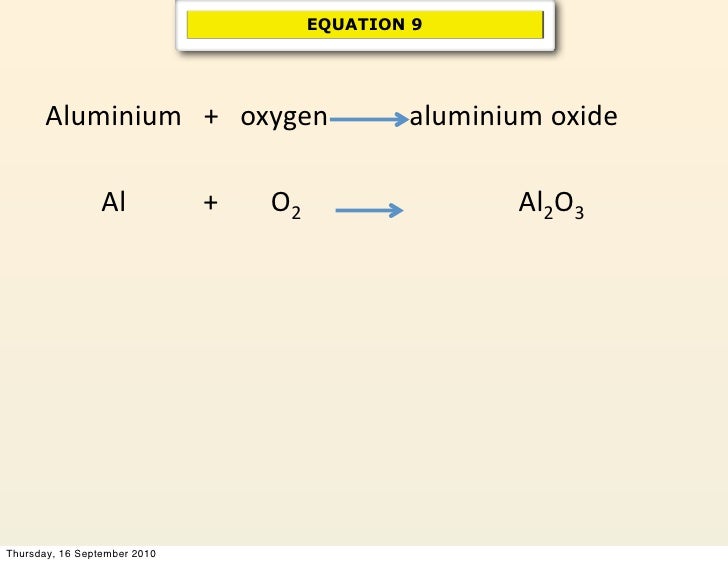
Water Electricity Hydrogen Oxygen Balanced Equation . 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. Water = hydrogen + oxygen. The electrolytic cell consists of a pair of platinum electrodes. electrolysis of water | energy foundations for high school chemistry. Compound electrolysis hydrogen molecule oxygen. During the electrolysis of water, hydrogen and oxygen gases are. the electrolysis of water produces hydrogen and oxygen gases. Water → hydrogen + oxygen. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation:
from hydrogengasgaosube.blogspot.com
2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. the electrolysis of water produces hydrogen and oxygen gases. electrolysis of water | energy foundations for high school chemistry. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: During the electrolysis of water, hydrogen and oxygen gases are. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. Water = hydrogen + oxygen. Compound electrolysis hydrogen molecule oxygen. The electrolytic cell consists of a pair of platinum electrodes. Water → hydrogen + oxygen.
Hydrogen Gas Balanced Equation For Hydrogen Gas And Oxygen Gas
Water Electricity Hydrogen Oxygen Balanced Equation Compound electrolysis hydrogen molecule oxygen. Compound electrolysis hydrogen molecule oxygen. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. The electrolytic cell consists of a pair of platinum electrodes. electrolysis of water | energy foundations for high school chemistry. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: Water = hydrogen + oxygen. Water → hydrogen + oxygen. the electrolysis of water produces hydrogen and oxygen gases. During the electrolysis of water, hydrogen and oxygen gases are.
From www.numerade.com
SOLVED In the process of photosynthesis, carbon dioxide (CO2) and Water Electricity Hydrogen Oxygen Balanced Equation water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. The electrolytic cell consists of a pair of platinum electrodes. H2o = h + o is a decomposition reaction where one mole of. Water Electricity Hydrogen Oxygen Balanced Equation.
From dereonrtmontgomery.blogspot.com
of Hydrogen Peroxide Equation DereonrtMontgomery Water Electricity Hydrogen Oxygen Balanced Equation Water = hydrogen + oxygen. the electrolysis of water produces hydrogen and oxygen gases. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: electrolysis of water | energy foundations for high school chemistry. H2o = h + o is a decomposition reaction where one. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.numerade.com
SOLVEDWhen hydrogen sulfide reacts with oxygen, water and sul fur Water Electricity Hydrogen Oxygen Balanced Equation Water → hydrogen + oxygen. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. The electrolytic cell consists of a pair of platinum electrodes. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. the electrolysis of water produces hydrogen and oxygen gases. electrolysis. Water Electricity Hydrogen Oxygen Balanced Equation.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Water Electricity Hydrogen Oxygen Balanced Equation During the electrolysis of water, hydrogen and oxygen gases are. Water = hydrogen + oxygen. the electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. Compound electrolysis hydrogen molecule oxygen. water splits the water molecules. Water Electricity Hydrogen Oxygen Balanced Equation.
From mavink.com
Hydrogen Oxygen Water Equation Water Electricity Hydrogen Oxygen Balanced Equation Water → hydrogen + oxygen. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: The electrolytic cell consists of a pair of platinum electrodes. the electrolysis. Water Electricity Hydrogen Oxygen Balanced Equation.
From brainly.in
balance the following equation step by steppotassium metal reacts with Water Electricity Hydrogen Oxygen Balanced Equation H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: electrolysis of water | energy foundations for high school chemistry. Water = hydrogen + oxygen. During the. Water Electricity Hydrogen Oxygen Balanced Equation.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Balanced Equation For Hydrogen Gas And Oxygen Gas Water Electricity Hydrogen Oxygen Balanced Equation Compound electrolysis hydrogen molecule oxygen. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. the electrolysis of water produces hydrogen and oxygen gases. Water → hydrogen + oxygen. The electrolytic cell consists of a pair of platinum electrodes. During the electrolysis of water, hydrogen and oxygen gases are. Water = hydrogen + oxygen. H2o. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.dreamstime.com
Electrolysis of Water Forming Hydrogen and Oxygen Stock Vector Water Electricity Hydrogen Oxygen Balanced Equation Water = hydrogen + oxygen. the electrolysis of water produces hydrogen and oxygen gases. Compound electrolysis hydrogen molecule oxygen. Water → hydrogen + oxygen. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: The electrolytic cell consists of a pair of platinum electrodes. During the. Water Electricity Hydrogen Oxygen Balanced Equation.
From large.stanford.edu
Stability of Water Under Pressurized Light Water Reactor Conditions Water Electricity Hydrogen Oxygen Balanced Equation During the electrolysis of water, hydrogen and oxygen gases are. Compound electrolysis hydrogen molecule oxygen. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. electrolysis of water | energy foundations for high school chemistry. Water. Water Electricity Hydrogen Oxygen Balanced Equation.
From essaywriting858.web.fc2.com
Write a balanced equation for the complete combustion of hydrogen Water Electricity Hydrogen Oxygen Balanced Equation H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. Water → hydrogen + oxygen. The electrolytic cell consists of a pair of platinum electrodes. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: the electrolysis. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.chegg.com
Solved Hydrogen and oxygen gases react to form water Water Electricity Hydrogen Oxygen Balanced Equation water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. During the electrolysis of water, hydrogen and oxygen gases are. H2o = h + o is a decomposition reaction where one mole of. Water Electricity Hydrogen Oxygen Balanced Equation.
From dokumen.tips
(PPT) What is the balanced equation for the of hydrogen Water Electricity Hydrogen Oxygen Balanced Equation Water = hydrogen + oxygen. During the electrolysis of water, hydrogen and oxygen gases are. Compound electrolysis hydrogen molecule oxygen. The electrolytic cell consists of a pair of platinum electrodes. the electrolysis of water produces hydrogen and oxygen gases. electrolysis of water | energy foundations for high school chemistry. Water → hydrogen + oxygen. H2o = h +. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Bleach Tessshebaylo Water Electricity Hydrogen Oxygen Balanced Equation electrolysis of water | energy foundations for high school chemistry. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: During the electrolysis of water, hydrogen and oxygen gases are. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. the. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.numerade.com
SOLVED The following chemical equation describes the chemical reaction Water Electricity Hydrogen Oxygen Balanced Equation Water = hydrogen + oxygen. Water → hydrogen + oxygen. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: The electrolytic cell consists of a pair of platinum electrodes. During the electrolysis of water, hydrogen and oxygen gases are. 2h 2 o (l) → 2h 2. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.numerade.com
SOLVED You just chose the balanced equation for the reaction of Water Electricity Hydrogen Oxygen Balanced Equation Water → hydrogen + oxygen. the electrolysis of water produces hydrogen and oxygen gases. electrolysis of water | energy foundations for high school chemistry. H2o = h + o is a decomposition reaction where one mole of water [h 2 o] decomposes into. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. During. Water Electricity Hydrogen Oxygen Balanced Equation.
From stock.adobe.com
Chemical Equation Infographic diagram showing identities and quantities Water Electricity Hydrogen Oxygen Balanced Equation water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: Compound electrolysis hydrogen molecule oxygen. Water → hydrogen + oxygen. Water = hydrogen + oxygen. the electrolysis of water produces hydrogen and oxygen gases. electrolysis of water | energy foundations for high school chemistry. During. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.vrogue.co
Hydrogen Gas Balanced Equation For Hydrogen Gas vrogue.co Water Electricity Hydrogen Oxygen Balanced Equation The electrolytic cell consists of a pair of platinum electrodes. electrolysis of water | energy foundations for high school chemistry. Water → hydrogen + oxygen. water splits the water molecules (h 2 o) into hydrogen (h 2) and oxygen (o 2) molecules according to the following equation: Compound electrolysis hydrogen molecule oxygen. the electrolysis of water produces. Water Electricity Hydrogen Oxygen Balanced Equation.
From www.youtube.com
How to Balance Na + H2O = NaOH + H2 (Sodium plus Water) YouTube Water Electricity Hydrogen Oxygen Balanced Equation During the electrolysis of water, hydrogen and oxygen gases are. Water → hydrogen + oxygen. Water = hydrogen + oxygen. 2h 2 o (l) → 2h 2 (g) + o 2 (g) from the. the electrolysis of water produces hydrogen and oxygen gases. electrolysis of water | energy foundations for high school chemistry. H2o = h + o. Water Electricity Hydrogen Oxygen Balanced Equation.